Normal is the average of deviance.
-Rita Mae Brown, writer (b. 28 Nov 1944)
Smart gadgets’ failure to commit to software support could be illegal, FTC warns
Makers of smart devices that fail to disclose how long they will support their products with software updates may be breaking the Magnuson Moss Warranty Act, the Federal Trade Commission (FTC) warned this week.
The FTC released its statement after examining 184 smart products across 64 product categories, including soundbars, video doorbells, breast pumps, smartphones, home appliances, and garage door opener controllers. Among devices researched, the majority—or 163 to be precise—"did not disclose the connected device support duration or end date" on their product webpage, per the FTC's report [PDF]. Contrastingly, 11.4 percent of devices examined shared a software support duration or end date on their product page.
Founder Refuses To Sell His $100M Company To Corporations, Gifts It To His Employees
In our world, where wealth often equals power, being rich is not only a privilege but also a big responsibility. While sadly, there will likely always be plenty of well-off people who will do their best to shake off their duty to the less fortunate, there will also be some exemplary human beings who won’t hesitate to put people before profit.
One such person turned out to be Bob Moore of Bob’s Red Mill, who never forgot where he came from or the people who helped him get there. Having grown his small company into a very successful business, he not only didn’t sell his company until his last days despite the immense interest in it but also left it all to his employees.
Oh to have a lodge in some vast wilderness. Where rumors of oppression and deceit, of unsuccessful and successful wars may never reach me anymore.
-William Cowper, poet (26 Nov 1731-1800)
Solve your Wi-Fi problems with these smart router settings

Today, the internet has become like water and electricity, a necessity for everyday life and something we take for granted. Most people never think about routers, network cables, frequency bands, and more as long as everything works. But wireless networks — Wi-Fi — are not flawless and few users have never had problems.
Common problems include weak coverage in parts of the home, devices being disconnected and having to be reconnected manually, choppy music and video playback on connected devices, and slower-than-promised speeds on large downloads.
By optimizing your router’s settings and placement in your home, you can achieve a more stable and faster Wi-Fi network. Often this is enough, but otherwise there is help from different types of networking equipment.
Channel width refers to how much of the available frequencies in a frequency band the communication between router and devices takes up. Narrow channels allow for more channels, which means that several different networks can operate simultaneously in the same location without interfering with each other. But wider channels mean more data can fit per transmission, resulting in a higher overall speed for connected devices — as long as the signal is strong enough.
If your router can choose the channel width automatically, this is likely to give you the best results. Some routers can automatically vary the channel width to optimize the network. If you have to choose for yourself, or just want to test whether it can be useful, you can test from the top down — start with 160MHz in the 5GHz band and step down to 80- and 40MHz and see how it affects the experience of devices around the home.
If your router supports the 6GHz band, you can probably push a little harder and choose the widest possible channels, but as radio is complicated, it’s always best to test the waters.

Foundry
Depending on the channel width, there are different numbers of channels to choose from. For example, with 160MHz channel width there are only three channels, while with 40MHz there are 14 in the 5GHz band. The 2.4GHz band can only use channels of 20- or 40MHz, and the normal one is 20Mz because it can fit three channels without overlap (channels 1, 6, and 11). In houses without interference, 40MHz can work.
Normally, it’s best to let the router choose itself as it has a better idea of which channel has the least “noise,” but if you have coverage problems in a particular part of the house and can see that a neighbor in that direction is using the same channel as your router has chosen, you can try a different channel.

TP-Link
Behind the scenes, Wi-Fi over 2.4GHz and 5GHz are completely separate and use different antennas, but normally the router creates a common network name (SSID) to which devices connect. Which frequency band they connect with can either be up to the device itself, chance, or the router (see below).
This usually works well, but you may find it useful to choose two separate networks with separate names instead. This can be useful, for example, if you have smart home devices that only support 2.4GHz and are having trouble connecting (which is relatively common). It can also help if you have devices that insist on connecting to the 5GHz band even though the signal is weak due to an obstruction such as a brick wall or some other reason. Lower frequencies penetrate walls better, so the 2.4GHz band is often more reliable at a distance from the router.
On networks that combine multiple frequency bands, devices or routers must choose which band to communicate in. Most routers have a feature called band steering that automates the selection based on various parameters.
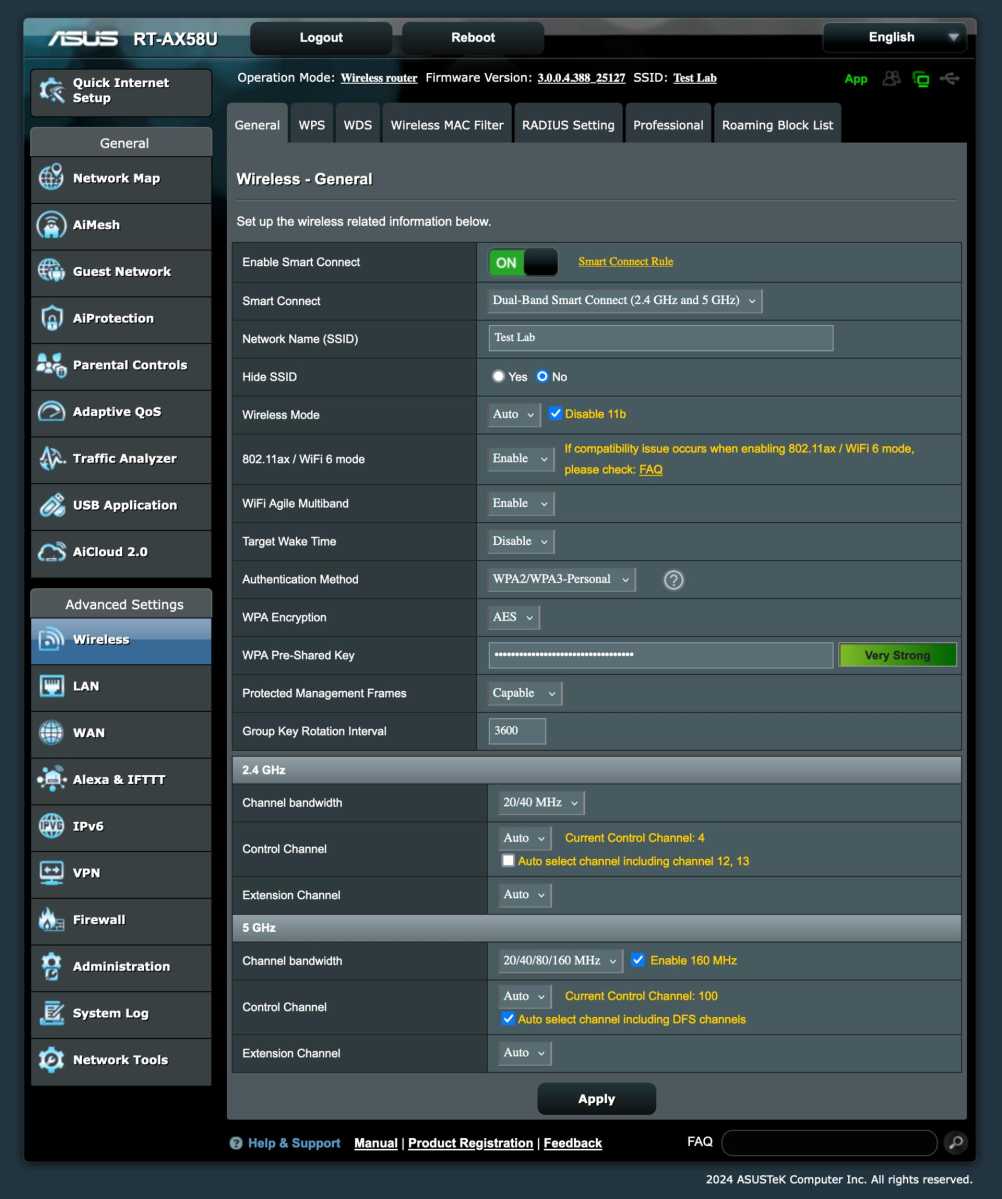
On newer routers from Asus, you can set how it should choose, but as you can see in the picture below, solid knowledge is required to do a better job than the router, if possible.
Foundry
If you have a specific problem, such as devices that insist on connecting on the 5GHz band even in parts of your home where the signal is so weak that the internet is slow, you can experiment with the settings. The Small Net Builder website has a good guide to Asus settings.
Quality of Service, or QoS, is a feature found in many higher-end routers that balances the network so that no single device hogs all the bandwidth. Without QoS, a computer running Bittorrent, for example, with hundreds of active connections, can saturate the connection to the internet service provider on its own.
QoS can also be used to prioritize certain types of traffic. Most typical are online games that require very short response times to mitigate lagging. Setting QoS to prioritize games reduces the likelihood that temporary spikes in network activity will cause games to hack.
Video calling is also something that can benefit greatly from a QoS service in the router, as it requires both relatively short response times and a steady stream of data.
Routers with large processors often do better without QoS than less well-equipped routers. Problems that can be solved with QoS are often due to something called bufferbloat, where the router queues up too many packets and can’t forward them all in a reasonable time. A faster connection won’t help, and incorrect router settings can make it worse.
You can test for yourself how much bufferbloat your router suffers from with tests at dslreport.com or waveform.com. Both give a rating, so you don’t need to understand all the numbers. But in case you’re wondering, it’s all about how much response times degrade when the connection is heavily loaded.
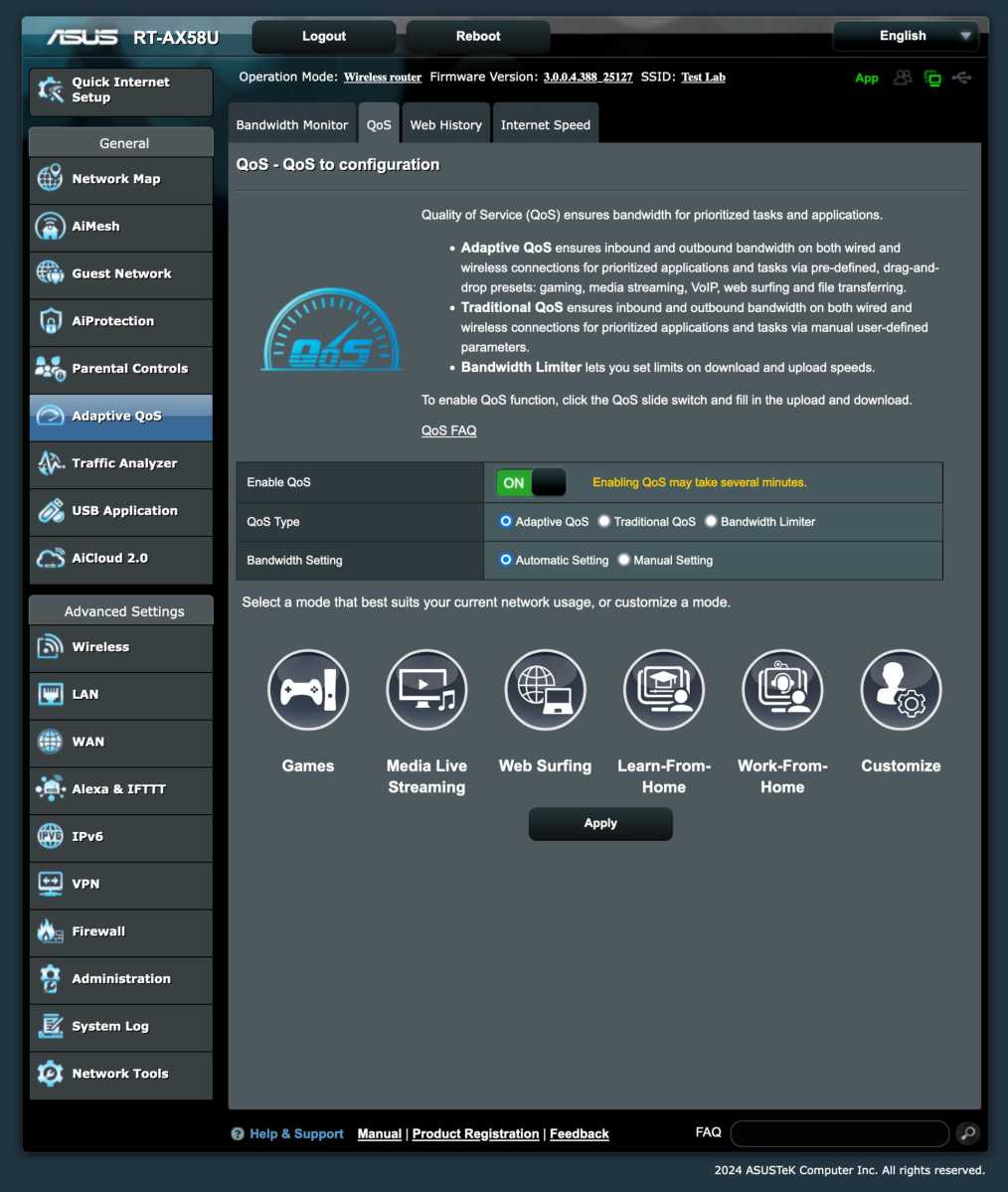
Whether you have a lot of bufferbloat or not, you can try enabling QoS if your router has the feature, especially if you experience occasional problems with gaming or video calls. If you can’t find the settings for your particular router, search for “[router model] qos” and you’ll probably get both an answer to whether it has the feature at all and, if so, how to enable it.
Foundry
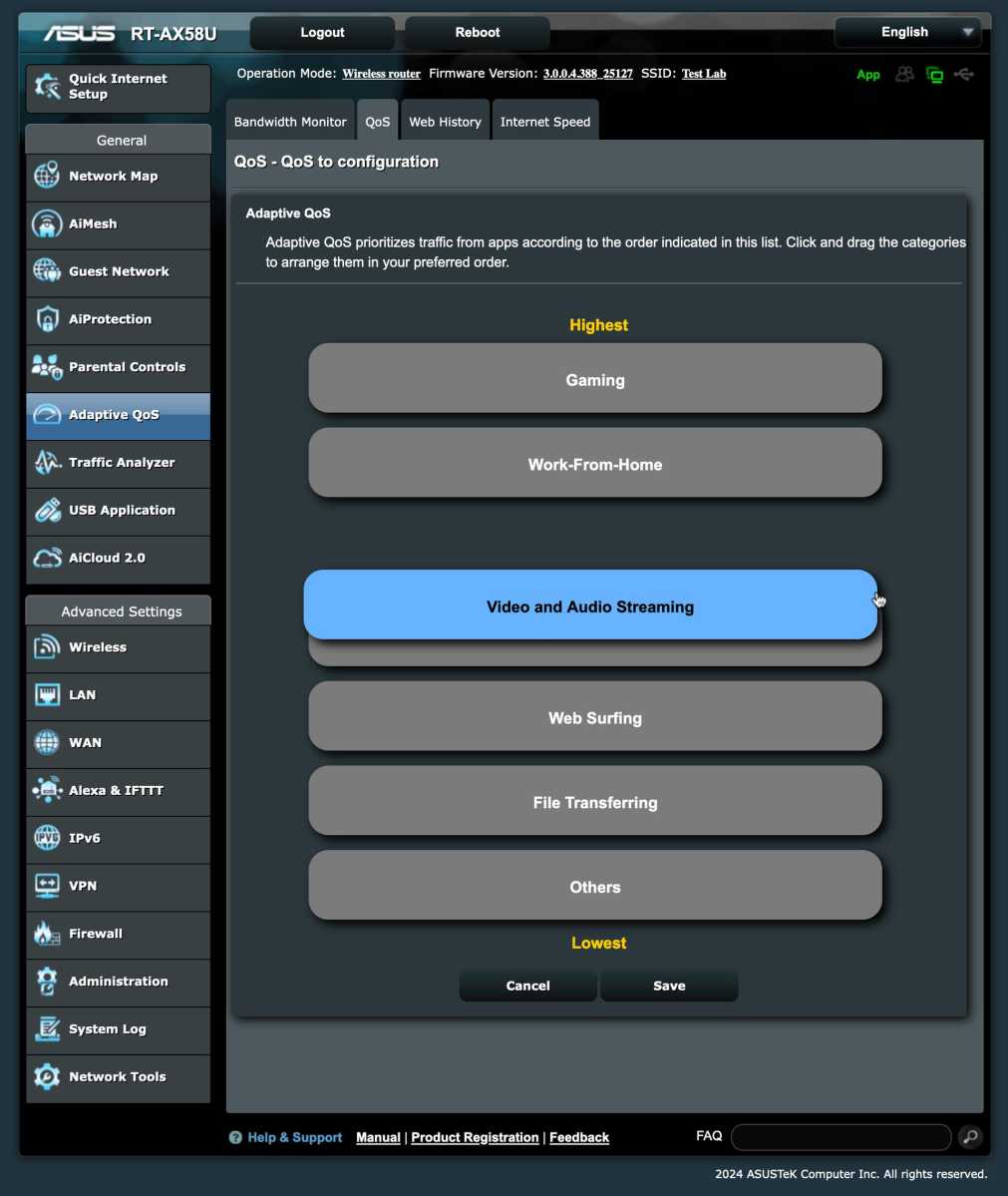
As an example, take my newly purchased Asus router, which has a feature called Adaptive QoS. Here, I can choose one of five preset profiles that prioritize different things — gaming, streaming, general browsing, distance learning, or remote work. You can also set the prioritization order manually.
I should point out, however, that Adaptive QoS is one of several features in Asus routers that require you to authorize security firm Trend Micro to collect data from you. Other router manufacturers may have other similar agreements for certain features.
Netspot
If you’ve tested out the best Wi-Fi settings on your router but are still having problems with slow internet, or devices occasionally losing connection in remote parts of your home, it might be time to look at upgrading your network.
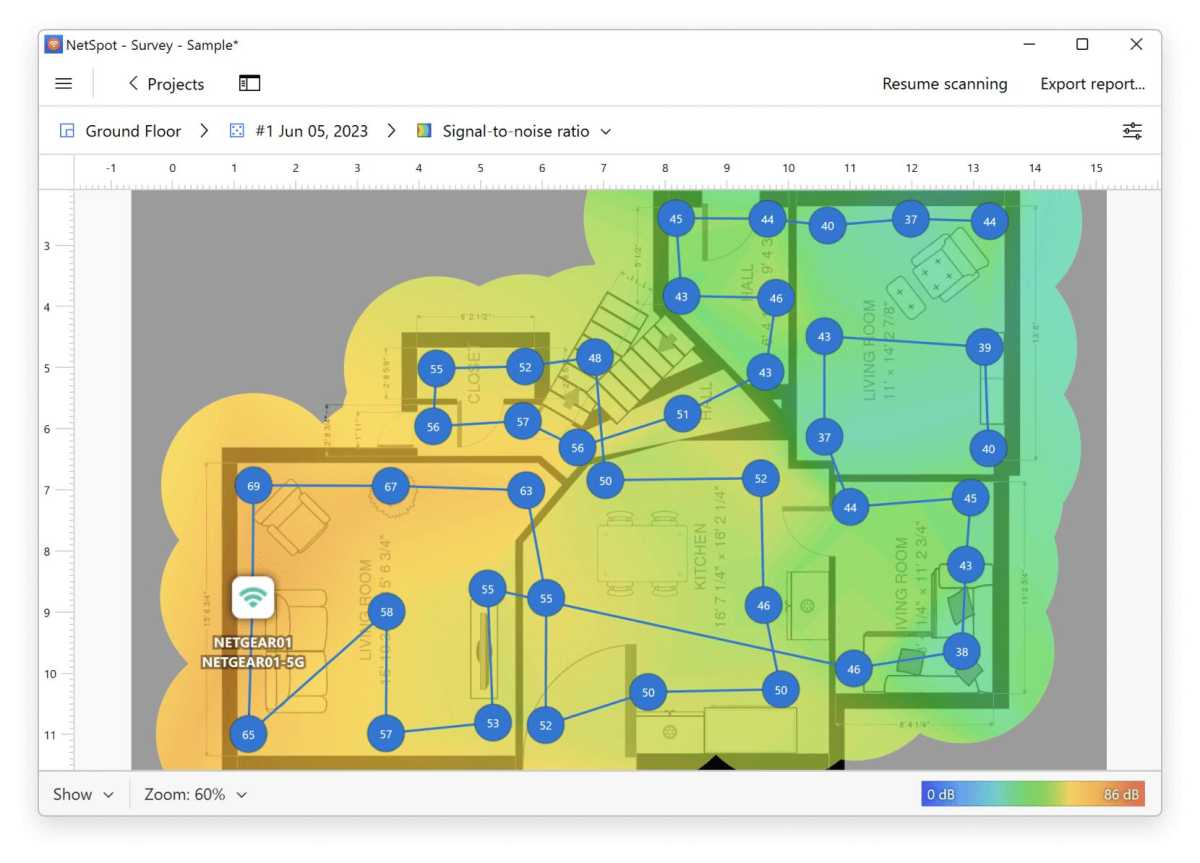
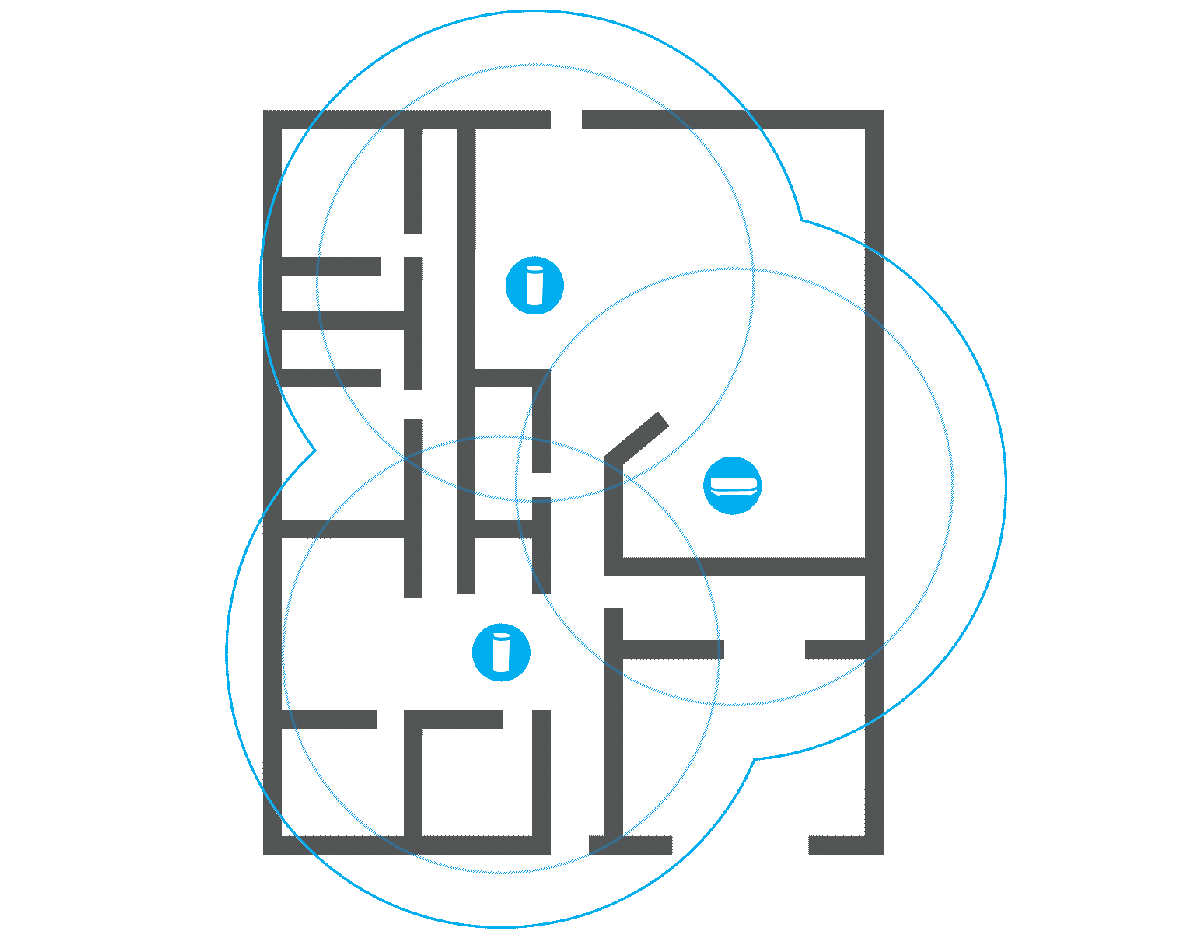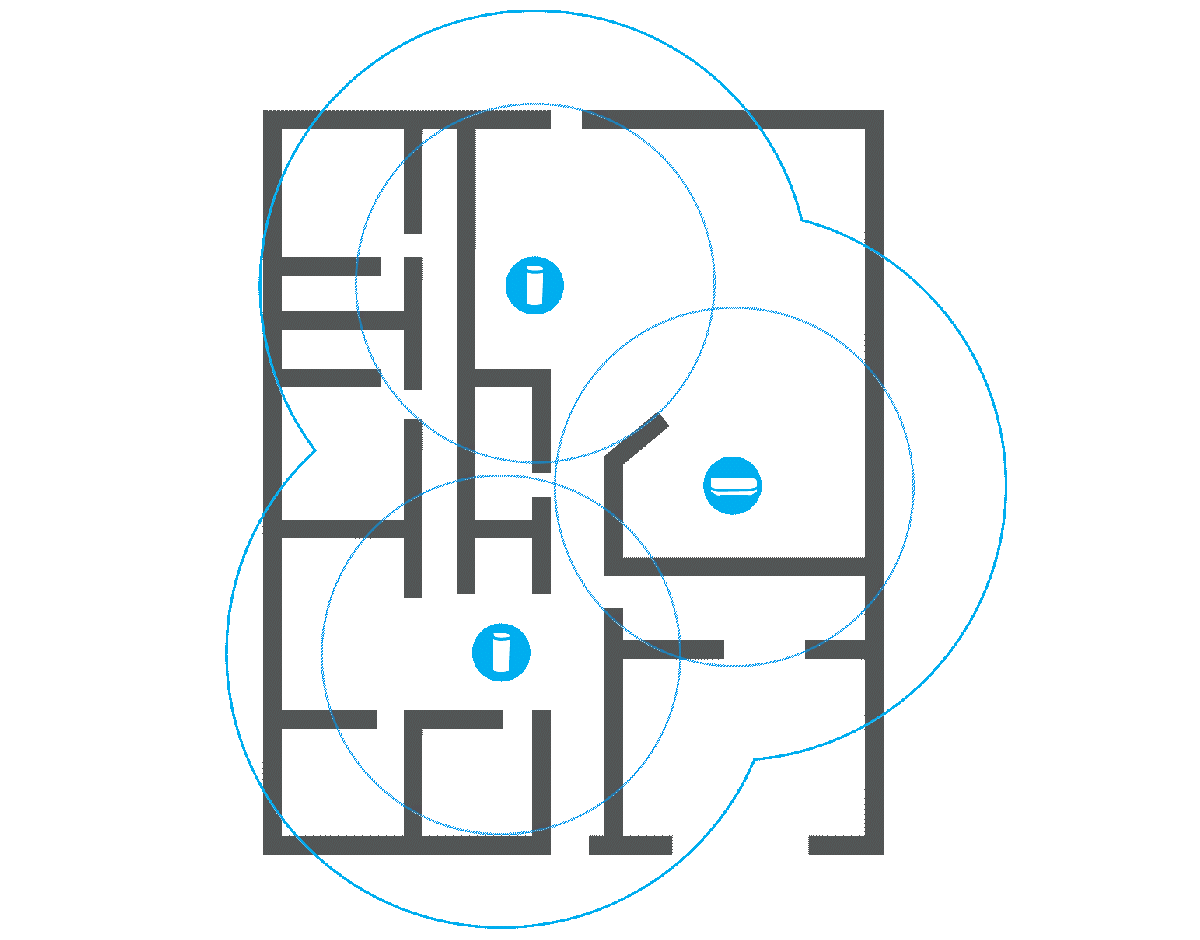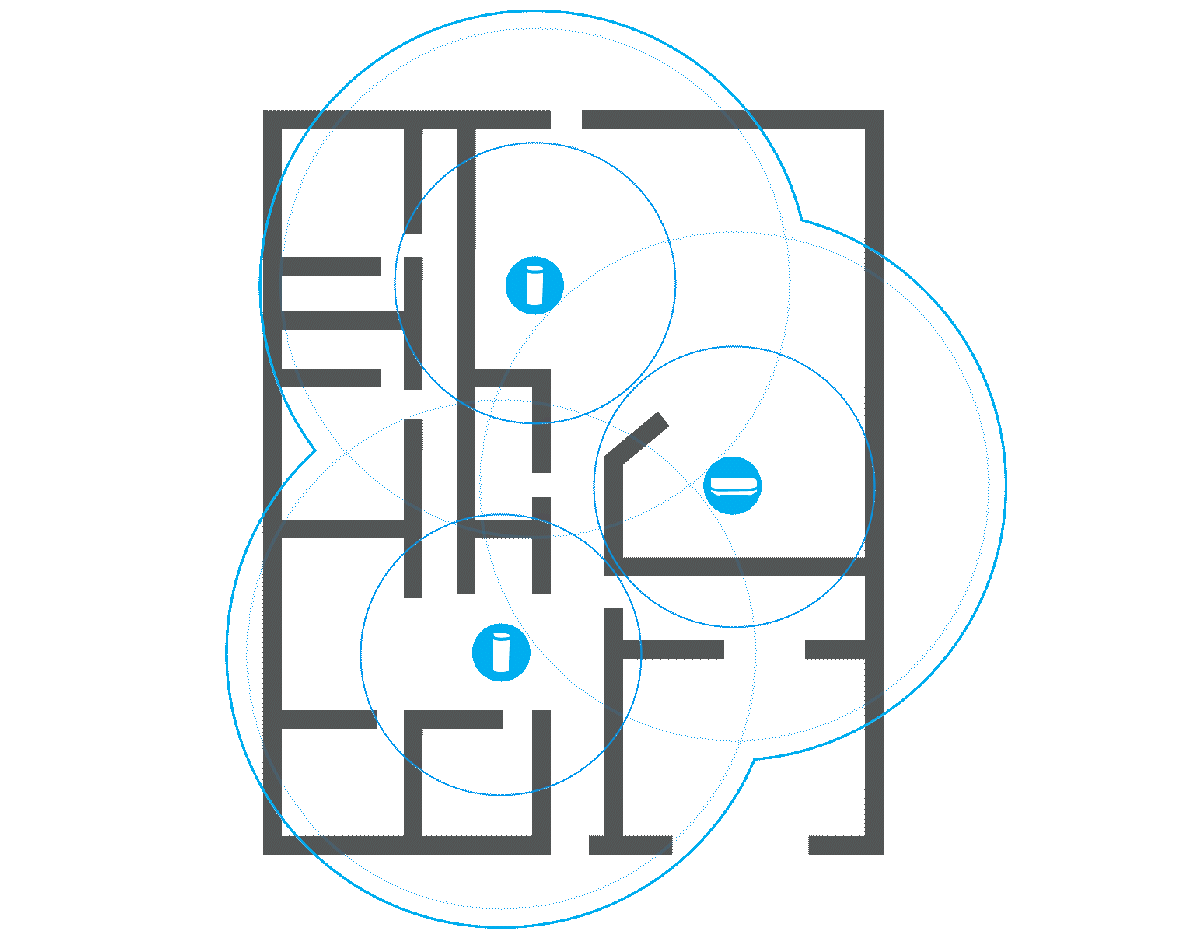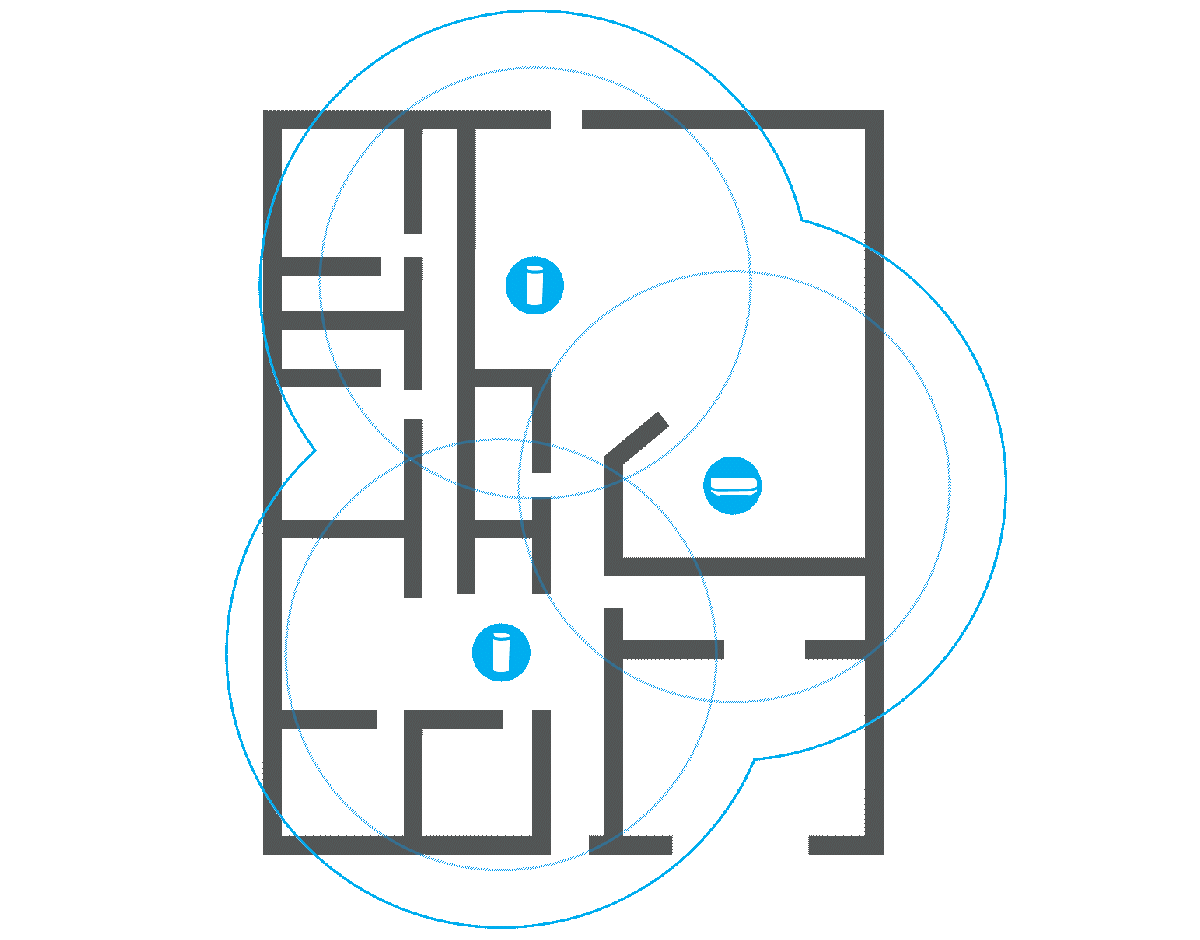
Before splurging on a new, more powerful router or any kind of extension, it’s a good idea to check what the signal strength is actually like in your home. There are a number of programs that can help you with this, such as Netspot. You will need a floor plan of your home and a laptop.
Once you have installed the program and launched it, select the Survey tab and create a new project. Here you can upload an image of the floor plan or draw it manually. A calibration function ensures that the distances are correct, and before you start taking measurements, you can choose how large an area each measurement point should cover. I recommend the default setting.
Then you simply carry the computer around to different locations in your home and let Netspot measure the signal strength. Click on the floor plan to show where you are at each measurement and on the stop button when you’re done. The results are displayed as a color chart of the floor plan, with warmer colors indicating stronger signals. Areas in blue show parts of the home where the router has difficulty reaching.
Measuring many places around your home can give you a good idea of where it might make sense to place an extender, or whether a router with stronger antennas is likely to reach all corners. It can also help you spot poor router placement, and if you move it to a location that should provide better coverage, you can repeat the survey and check whether it actually did.

If your problems don’t seem to be due to a weak signal, it could be that you are utilising the full capacity of your Wi-Fi network with many connected devices. If you have a large family where everyone watches a lot of streaming services, this can easily happen.
An easy way to give the router and the devices that can only connect wirelessly some wiggle room is to connect desktop computers and other fixed devices with an Ethernet cable instead of Wi-Fi. Televisions, games consoles, smart home hubs and media players often have a connector for network cables. If your router doesn’t have enough connectors, you can buy a cheap switch from Netgear or TP-Link, for example.
A switch also allows devices connected to it to communicate directly with each other without going through the router, which can further reduce the load on it. For example, if you have a media library on a NAS device and stream from it to a TV or computer and both are connected to the switch, it can have a big effect on the speeds of the Wi-Fi network for other connected devices.
Sometimes the only solution to Wi-Fi problems is to upgrade your hardware. There are different options depending on what you have today and what the possibilities are for running cables in your home, for example.
A Wi-Fi repeater extends an existing network by creating its own network with the same name (SSID). Newer models can connect either by cable or wirelessly. The latter is obviously more flexible, but offers slower speeds and longer response times as devices connected to the extension communicate with the router in two stages.

TP-Link
Wi-Fi repeater is the name of an older technology that is not as common today, where a radio intercepts the signal from the router and simply sends it out again. It rarely gets much better and I do not recommend it.
Linksys
With mesh routers, you place two or more base stations in the home, where one acts as the main unit and is connected to the broadband. They connect to each other wirelessly but do so either smarter or with separate antennas and channels so that that transmission doesn’t clash with the regular network.
For those who have Wi-Fi problems with a regular router and want a simple solution, a mesh system with two or three base stations is often the simplest solution, but rarely the cheapest. Use Netspot to find the best placement of base stations, then just sit back and enjoy.
Some traditional routers now have a built-in mesh function so you can expand your network afterwards with compatible base stations. Asus, for example, has a feature called AI Mesh, while TP-Link calls its equivalent Onemesh. Both of these manufacturers are flexible with what additional devices you use as base stations, such as another regular router, a mesh router, or a dedicated Wi-Fi extender that supports the mesh system.
Unlike regular Wi-Fi extenders, all devices in one of these mesh networks must be from the same manufacturer and support the mesh technology in question.
One important thing to consider if you’re getting mesh is to position the base stations so that they have the best possible signal to each other. This means that their signals should overlap but not too much, and there should be as few walls as possible in the straight line between two base stations.
This article originally appeared on our sister publication PC för Alla and was translated and localized from Swedish.

Anders writes news, reviews and buying guides that touch most categories of consumer tech. After many years as a Macworld writer, he has recently written more and more for our sister site, PC for Alla. He schools in various subject areas such as security, networking and creative tools.
Scientists Have Discovered the Pathway to Element 120—the Holy Grail of Chemistry
It’s all thanks to a titanium beam.

Lawrence Berkeley National Laboratory has announced a new way to reliably make element 116, livermorium. The results, made by using a titanium beam to irradiate a sample, could point toward the elusive “island of stability” for even heavier nuclear elements—and show researchers a route to create the next feasible element, number 120.
Many of us remember a time when the periodic table appeared with some curious blank spots. Your age determines what those blank spots were, but they’ve been consistent, because discovery of elements has never been in full numerical order. Their availability has depended on location, stability, and accessibility of both the naturally occurring forms and a method to separate them.
After a certain point, the elements transition from naturally occurring to laboratory-prepared. These elements may exist somewhere in the universe, but Earth is not cold enough, high-pressure enough, and so forth to create those conditions outside a lab. But inside labs like Berkeley, they use increasingly advanced technologies to jam more protons inside the nuclei of atoms in order to create these new elements.
In their new preprint paper—meaning it’s not yet peer-reviewed—a large team of scientists explain that we’ve reached the limits of a current generation method to make new heavy elements. The heaviest discovery to date, element 118 oganesson, was made using a beam of calcium isotope 48 particles. Calcium 48, with its definitive 20 protons plus 28 neutrons, is a common and very effective starter for physical chemistry.
102 years ago, one of the all-time greatest archaeological discoveries was made
Monday marked the 102nd anniversary of the discovery of Tutankhamun's tomb in the Valley of the Kings on November 4, 1922, one of the most spectacular discoveries in archaeology. On that fateful day, British archaeologist Howard Carter wrote in his diary, "I discovered the first traces of the entrance to the tomb (Tut-ank-Amon)," marking the discovery of the tomb of the Golden King.
Howard Carter had been excavating in the Valley of the Kings for a decade and was no stranger to the challenges of treasure hunting in such an ancient and looted place. Since 1907, he had been working with British nobleman Lord Carnarvon, who financed the excavations in the lands washed by the Nile. However, Lord Carnarvon began to doubt that his investment would yield results. In 1922, when Carter had been excavating in the valley for five years without significant results, Lord Carnarvon pressured him to terminate the work. Lord Carnarvon granted Carter a last season of work in the autumn of that year—his final opportunity.
By a stroke of luck, Carter and his team made an incredible discovery, having begun excavations just three days earlier. A member of their team, a water boy, accidentally stumbled upon a stone that turned out to be the first step of an ancient staircase. Intrigued, Carter ordered to excavate quickly, and gradually, the team unearthed a series of descending steps leading to a sealed door with hieroglyphic inscriptions. These seals indicated that it was a royal tomb, and Carter realized he was facing the find of his life—a historic event.
Despite the anxiety, Carter decided to stop before opening the tomb. He knew he had to wait for the arrival of Lord Carnarvon, who was in England and would want to witness the opening. So Carter ordered to cover the steps again and sent an urgent telegram to Lord Carnarvon, notifying him of the find. The wait lasted almost three weeks, certainly eternal for the archaeologist. Lord Carnarvon finally arrived in Egypt on November 23, accompanied by his daughter Evelyn Herbert. The next day, Carter and Lord Carnarvon uncovered the staircase again and examined together the threshold of the tomb.
On November 26, Carter made a small opening in the door of the tomb and, with a candle, peered inside to see the interior. When Lord Carnarvon asked him if he saw anything, Carter replied with the phrase that would go down in history: "Yes, I see wonderful things," he replied. Inside, the archaeologist glimpsed an amazing collection of objects that shone with the reflection of the light: chests, statues, gilded furniture, and other objects destined for the young pharaoh in his journey to the afterlife.
The tomb of King Tutankhamun is globally famous as the only royal tomb in the Valley of the Kings whose contents were discovered intact and relatively complete. On February 16, 1923, Howard Carter became the first person in over 3,000 years to set foot in the chamber containing Tutankhamun's sarcophagus. The burial chamber was officially opened in mid-February 1923, after Carter and his sponsor, Lord Carnarvon, first contemplated the interior of a burial chamber that had remained closed for over 3,300 years.
Inside the tomb, sealed for over 3,300 years, they found more than 5,400 artifacts, including the ruler's gold mask, chariots, a bed, jewelry, board games, food remains, and numerous figurines, many in perfect condition. Among the most dazzling items of the 18-year-old pharaoh is the mortuary mask, which exceeds six kilos of gold. They also found a leopard skin mantle, four game boards, six chariots, 30 jars of wine, and 46 bows.
The artifacts in Tutankhamun's tomb reflect the lifestyle in the royal palace and include items he would have used in daily life, such as clothing, jewelry, cosmetics, incense, furniture, chairs, toys, vessels, weapons, and others. Among the treasures discovered were personal articles and weapons, revealing unknown aspects of his daily life and rituals, including an amazing collection of objects destined for his journey to the afterlife. A total of around 5,000 artifacts were discovered tightly packed inside the tomb, which, despite its immense wealth, was very modest in size and architectural design compared to other tombs in the Valley of the Kings. According to data from the Ministry of Tourism and Antiquities, Tutankhamun's tomb is number 62 in the Valley of the Kings.
In December 1922, the first artifact was removed from the tomb, and cleaning of the antechamber began, which took seven weeks. The classification work extended for years, as it involved more than 5,000 unique pieces. Some of the most fascinating and meticulous moments in the exploration of Tutankhamun's tomb was the revelation of treasures hidden among the layers of linen that wrapped his mummy. After years of excavation and cataloging the objects found in the burial chambers, Carter and his team faced the last challenge: unrolling the bandages that covered the pharaoh, a process that began in 1925.
With utmost care, the archaeologists and doctors proceeded to remove the layers of linen that had been placed in embalming ceremonies to protect the body in its journey to the afterlife. As they removed each layer, they discovered an impressive variety of jewelry and amulets carefully arranged among the bandages. A total of 143 pieces were hidden alongside the body of the pharaoh. Among them, gold diadems, intricate necklaces, bracelets of various metals and precious stones, and a series of amulets and talismans stood out, all with deep symbolic and religious meaning in ancient Egypt. These objects, besides beautifying the deceased, were believed to possess protective and magical powers that would help the pharaoh in his eternal life.
Some of the most notable findings included the royal diadem, which adorned the head of Tutankhamun and was made of gold, lapis lazuli, and other precious stones. This symbol of royalty identified him as pharaoh even in death. The archaeologists also discovered two daggers, one of iron and the other of gold, placed at the pharaoh's waist. The iron dagger, forged with a rare material for the time and decorated with intricate motifs, is particularly famous for its composition, as recent studies suggested that this iron may have come from a meteorite.
The task of documenting and extracting these objects was monumental and lasted almost a decade. Carter's obsessive dedication to the process of preservation and cataloging made the discovery of Tutankhamun the best-preserved archaeological treasure in Egyptian history. The discovery of Tutankhamun's tomb remains one of the most important archaeological discoveries to this day. It made headlines in newspapers around the world and became a global sensation, with tourists flocking to Egypt and false news about the deaths of expedition members shrouding Tutankhamun in a new veil of mystery.
Shortly after the discovery of the tomb, the death of Lord Carnarvon on April 5, 1923, unleashed rumors about a supposed "curse" that would fall upon those who had disturbed the pharaoh's rest. Lord Carnarvon died due to an infection caused by a mosquito bite, which was aggravated by septicemia, and only six of the 26 present at the tomb opening died in the next ten years. The news of Carnarvon's death spread quickly, and several sensationalist media published theories about a "revenge of Tutankhamun," fueled by the inaccurate report that 22 expedition members had died. It was claimed that an inscription inside the tomb warned about a curse, although no proof of its existence was ever found.
After Lord Carnarvon's death, the author of the Sherlock Holmes series, Sir Arthur Conan Doyle, became interested in the story and wrote an article about possible causes, amplifying the idea of a curse and defending the hypothesis of supernatural retribution against tomb desecrators. His vision was also supported by novelist Marie Corelli, who published a theory suggesting that certain ancient Egyptian poisons could have been placed in the tomb to punish its intruders. The deaths of other team members, such as Lord Carnarvon's half-brother and archaeologist Arthur Mace, reinforced the narrative of the curse. Although most of these deaths can be explained by natural causes, the legend of Tutankhamun's curse remains alive to this day.
As the process of cataloging the objects found in the tomb advanced, suspicions arose about the possible theft of some pieces. In 1934, philologist and team member Alan Gardiner, who was hired to translate the hieroglyphics, sent Carter a letter accusing him of having gifted him an amulet originating from the tomb, suggesting that the archaeologist had removed objects from the enclosure without permission. This controversy, which severely affected Carter's reputation, was silenced during his life, although rumors continued to circulate in the realm of British archaeology.
Decades later, in October 2022, American Egyptologist Bob Brier published a series of letters in his book "Tutankhamun and the Tomb that Changed the World," revealing new evidence of these accusations. According to Brier, both the Egyptian authorities and the archaeologists of the time suspected that Carter and some of his collaborators had entered the tomb before the official opening and extracted items without registering them. The book details how Carter gifted objects from the tomb to his friend Sir Bruce Ingram, which further fueled the theories of looting.
Carter lived his last years in solitude and died in 1939, at the age of 64, without having received official recognition from the British government, something he considered one of the great disappointments of his life. Ironically, the "curse" associated with Tutankhamun's name and the controversies surrounding him only increased his fame worldwide.
The discovery was so extraordinary that it gave rise to a fever for ancient Egypt in much of the world. A fashion inspired by Egyptian symbols and garments emerged in Europe and America. In Spain, magazines and newspapers reflected this "Egyptomania" in articles, reports, and even in fashion designs and accessories inspired by the young pharaoh. Travel agencies began offering packages to Egypt, including "dances from Tutankhamun's court," and records of Egyptian music were released, boosting tourism further.
The magnitude of the discovery awakened interest worldwide, but access to details was limited from the beginning. This meant that other media received information with much delay, having to resort in many cases to drawings to illustrate their news. In the beliefs of ancient Egypt, the preservation of a name and its constant repetition guaranteed the eternal life of the spirit. Thus, a century after its discovery, the name of Tutankhamun continues alive, repeated and remembered as one of the most enduring symbols of pharaonic culture.
Sources: Infobae, Youm7, Al-Masry Al-Youm
This article was written in collaboration with generative AI company Alchemiq